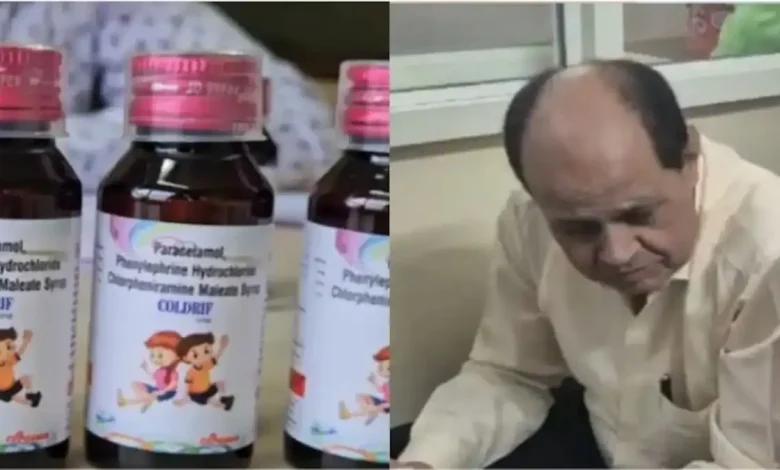
BHOPAL: Hours following his detention, health authorities in Madhya Pradesh suspended Dr Praveen Soni, a pediatrician serving at Parasia’s Civil Hospital in Chhindwara district, after 11 children lost their lives to kidney failure caused by tainted Coldrif cough syrup.
Health Commissioner Tarun Rathi issued the suspension order on Sunday, noting that Dr Soni was operating a private practice where he prescribed medications to infants seeking treatment. The order detailed how children who consumed these prescribed medicines subsequently experienced severe symptoms including high fever and urination difficulties, with several cases proving fatal.
According to the inquiry findings documented in the suspension order, the medicines prescribed by Dr Soni adversely impacted the kidneys of these young patients, ultimately leading to their deaths. The investigation revealed a disturbing pattern: children developed acute symptoms only after taking the doctor’s prescribed medications during his private consultations.
The official report emphasized that proper medical examination, accurate diagnosis, and appropriate treatment protocols could potentially have prevented these tragic deaths. However, Dr Soni allegedly failed to provide adequate care during his private practice sessions.
The suspension order states that through his actions, Dr Soni inflicted permanent damage, damaged the department’s reputation, and demonstrated an inability to meet his professional obligations and duties.
Laboratory analysis of the Coldrif syrup revealed alarming levels of diethylene glycol (DEG) — a toxic industrial compound commonly found in antifreeze and brake fluid products — ranging from 46.28% to 48.6%. The fatalities occurred throughout the previous month, predominantly affecting children younger than five years.
Treatment efforts at Nagpur’s Government Medical College and Hospital could not save ten of the affected children, while six others continue receiving medical care at the facility.
State authorities have prohibited the distribution of Coldrif syrup and directed drug inspectors to confiscate existing inventory while testing additional batches from Sresan Pharmaceuticals, the Tamil Nadu-based manufacturing company.
Chief Minister Mohan Yadav declared financial assistance of Rs 4 lakh for each bereaved family and committed state funding for ongoing medical treatment of surviving affected children.
An FIR has been registered at Parasia police station against Dr Soni and Sresan Pharmaceuticals under provisions of the Bharatiya Nyaya Sanhita and the Drugs and Cosmetics Act. The complaint was filed by Dr Ankit Sahlam, Block Medical Officer at the Community Health Centre in Parasia, supported by medical documentation and laboratory evidence confirming acute kidney injury in the victims.
Officials have indicated that investigations continue, examining the complete supply chain and accountability measures regarding the distribution and prescription of the contaminated medication. The Central Drug Standards Control Organisation has launched multi-state inspections covering pharmaceutical facilities across Madhya Pradesh, Maharashtra, and Tamil Nadu, with particular attention to cough syrups, fever-reducing medications, and antibiotic formulations.
Victims of the Tragedy:
- Shivam Rathore (4) – September 4, 2025
- Vidhi Namita (3) – September 5, 2025
- Adnan – September 7, 2025
- Usaid (4) – September 13, 2025
- Rishika (5) – September 15, 2025
- Hitansh Soni (5) – September 19, 2025
- Chanchlesh – September 26, 2025
- Vikas – September 26, 2025
- Sandhya – October 1, 2025
- Yogita Thakre (2) – October 4, 2025
Authorities have confirmed the investigation remains active, with primary emphasis on establishing accountability for all parties involved in manufacturing, distributing, and prescribing the toxic medication.




