A central team from the National Centre for Disease Control (NCDC) is investigating the suspected role of cough syrups in the recent deaths and illnesses of children in Madhya Pradesh and Rajasthan. The probe has led to the urgent testing of specific syrup batches and a halt in their distribution.
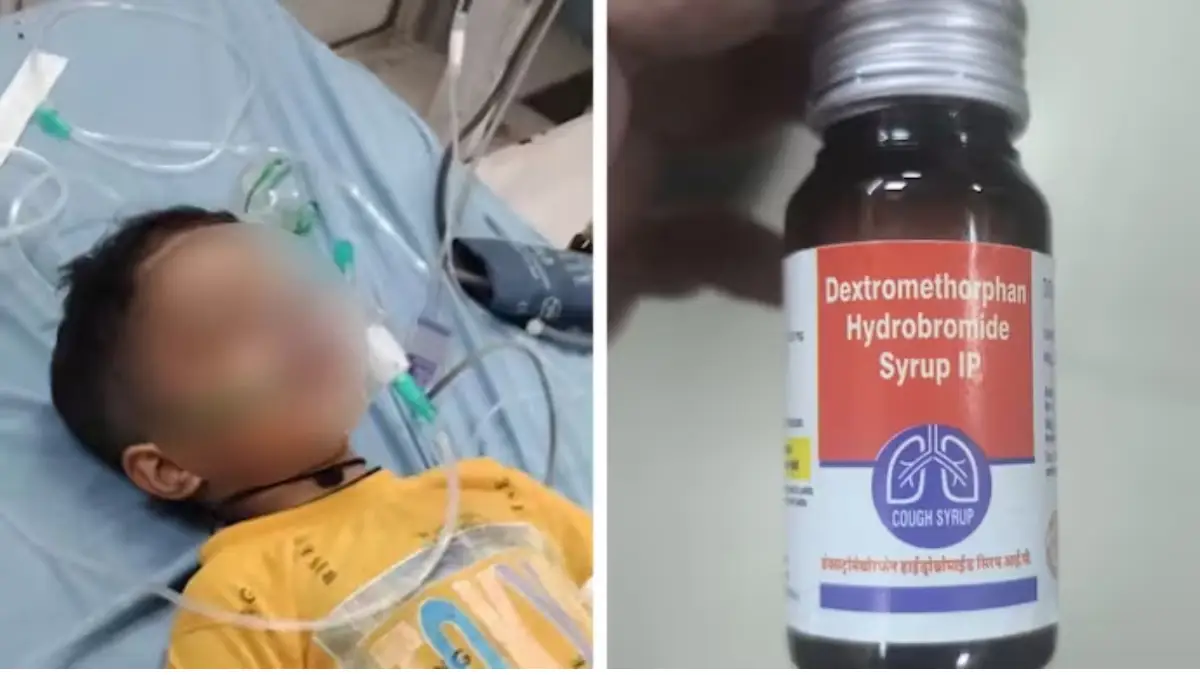
The investigation was triggered by a series of alarming incidents. In Rajasthan, a five-year-old boy in Sikar died after consuming a cough syrup supplied under the state’s free medicine scheme. A three-year-old in Bharatpur also fell seriously ill after taking the same syrup. Separately, in Madhya Pradesh’s Chhindwara district, six children have died over the past month from suspected kidney infections after reportedly consuming similar medications, prompting a local ban on Coldrif and Nextro-DS Syrups.
Authorities are analyzing multiple samples, including water and drugs, to rule out infectious diseases, but the quality of the cough syrups has become the primary focus.
In Rajasthan, the incidents involve Dextromethorphan Hydrobromide syrup supplied under the Chief Minister’s Free Medicine Scheme. A two-year-old girl in Sanganer was hospitalized in critical condition after being administered the syrup. This case follows earlier illnesses in Bharatpur and Sikar, where several children were hospitalized but later discharged. Notably, a government doctor in Bharatpur also reported adverse symptoms after using the syrup himself.
In response, the Rajasthan Medical Services Corporation Limited (RMSCL) has suspended the distribution of batches KL-25/147 and KL-25/148, manufactured by Jaipur-based Kaysons Pharma. A three-member committee has been formed to investigate. RMSCL officials noted that over 133,000 patients had received this syrup since June with no prior complaints, but distribution has been suspended as a precaution.
Rajasthan Drug Controller Ajay Phatak confirmed that the entire supply of the syrup has been suspended pending test results, with a detailed report expected within days. Initial observations suggest the syrup may not have been suitable for paediatric use, raising concerns about prescription practices under public health schemes.
In Chhindwara, the NCDC has collected samples, and test results from State Drug Testing Laboratories are pending. Health experts are now advising against the unsupervised use of over-the-counter cough medicines for children, emphasizing that parents should not administer any medication without a doctor’s prescription, especially for children under five. The distribution of the implicated batches remains suspended as authorities await lab results.